by Leland Teschler, Executive Editor
Hydrogen fuel cells have numerous advantages over the best battery technology for running planes and even heavy duty vehicles.
Batteries grab headlines these days, but it increasingly looks as though the power source likely to have the most impact on vehicle technology in the future will be the hydrogen fuel cell. That is the conclusion pointed to by recent developments on the alternative fuel front.
For example, consider what’s going on in aerospace: European budget airline EasyJet PLC plans to test fuel cells on its Airbus planes as a means of taxiing without running the main engines. The airline figures to save on jet fuel this way. At the U.S. Naval Research Lab, fuel cells have been powering various drones since 2009. Researchers there say they are taking advantage of advances coming out of fuel cell research in the auto industry: NRL cells use the same proton-exchange membrane material as found in the Toyota Mirai, a fuel cell vehicle released in 2014 that gets 66 mpg and runs for 312 miles on a full tank of hydrogen.
There have been several developments in fuel-cell-powered drone aircraft. Intelligent Energy Ltd. In the UK has attached a hydrogen fuel cell to a $3,200, 7.5-lb quadcopter made by DJI in China. The fuel cell and its fuel weigh just 3.5 lb, which is lighter than the battery it replaces. On another front, researchers at the Pohang University of Science and Technology in South Korea have created a miniature fuel cell targeting drones and similar devices they say has a peak power density of around 560 mW/cm3. And fuel-cell developer EnergyOr Technologies Inc. in Montreal has integrated fuel cells into several drones, including one developed by Israel Aerospace Industries. The company has also kept a 20-lb winged UAV called the Faucon H2 in the air on fuel-cell power for ten straight hours.
Of course, the use of fuel cells to power relatively light drones could be considered technological baby steps. Fuel cells are still considered expensive. The development that would go the farthest to reduce their cost would be their adoption as a mainstream power source in high-volume vehicles. It looks as though that could begin to happen in about four years.
So says Charles Freese, General Motors executive director for global fuel cell activities. GM has developed several fuel cell vehicles since the late 1960s. In 2008, it fielded 120 fuel-cell powered Chevy Equinox SUVs that GM says have accumulated nearly three million driving miles. General Motors has also agreed to build all-terrain vehicles powered by hydrogen fuel cells for the U.S. Army and is working closely with Honda as a way of more rapidly getting to large-scale deployment of fuel cell technology. But Freese doesn’t see big fleets of fuel-cell vehicles on the road until about 2020.

The argument for fuel cells
The reason automakers are interested in fuel cells becomes clear by comparing fuel-cell energy density to that of batteries.
“We look at volumetric and gravimetric energy storage density as figures of merit. Gasoline and diesel fuel tend to operate in the 30 to 40 Mj/l and 30 to 35 Mj/kg range. Hydrogen comes in at a little under 10 Mj/l and 10 Mj/kg. But those values are orders of magnitude better than the best lithium-ion batteries,” says Freese. “That’s why hydrogen is so interesting. It can keep up the range for larger vehicles and still allow refueling in three minutes. The down side is that battery powered vehicles are 92 to 93% efficient from tank to wheel. That figure is about 65% for equivalent fuel cell vehicles.”
Those efficiencies are probably about as good as they’re going to get for the near future, Freese thinks. The emphasis now is on getting the cost of the fuel cell down rather than on improving power density and efficiency.
“Initially, technological development efforts in fuel cells were aimed at ensuring they weren’t excluded from powering vehicles because of the packaging or because they didn’t provide enough power. Those obstacles were overcome quite some time ago and they no longer guide our work,” says Freese. “We continue to push for smaller systems. For example, our current generation of fuel cells are about half the size and half the mass of those in the Chevy Equinox SUVs on the road since 2008. But now primarily we are trying to take cost out.”
An example of GM’s cost reduction efforts can be found in the platinum catalyst used to promote oxidation reactions that generate positively charged hydrogen ions and electrons in the cell. “Platinum was a key cost driver for a long time,” says Freese. “The Equinox system had 80 gm of platinum. Now we are making cells that use 27 gm. And we are working on the next generation which will use only 7 to 12 gm. When you get that low, platinum is no longer a major cost driver. The amount of precious metal in the after-treatment systems of big diesels can approach the level you see in fuel cells.”
Intense system integration also gets credit for reducing costs. “The Equinox fuel cell needed seven injectors to inject hydrogen. Now we are down to one. The old designs also had a large manifold and controller. Now we use a controller that is size of your pinky finger that integrates into an existing manifold,” Freese says.
There have also been advances in the construction of the plates inside the cell that separate the hydrogen, oxygen, and cooling components. “In our first generation, those plates were a composite. It was brittle, thick and expensive. They were also porous enough to let molecules migrate through over time. Now we’ve gone to a thin, stamped stainless-steel plate that harkens back to our automotive roots. The plates are foil-like and robust and look a lot more like a head gasket in an engine,” Freese says.
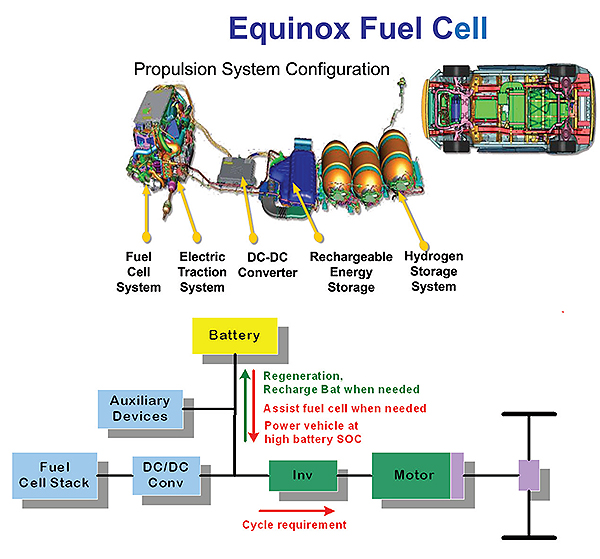
Similarly, GM has found ways to mass-produce fuel cell proton-exchange membrane material. “The technique we use borrows much from the days of Kodak producing photographic film on roll-to-roll machines. And the way we process catalysts is much like the way photocopying inks are prepared,” says Freese.
Still, there are operational areas associated with fuel cells that need some work. “Heat rejection and getting enough radiator space is part of the challenge,” says Freese. “We are not happy with where we are today, and we are pushing in that area. But the good thing is that the levels of heat we are dealing with are not stressing the system thermally or mechanically. We run in the 95°C range and lower sometimes, compared to the 700°C you have in conventional exhaust temperatures. So the delta-T is actually smaller than in an internal combustion powertrain.”
Automakers also want fuel-cell-powered vehicles to last as long as those powered conventionally. But the wear-out mechanisms for a fuel cell are totally different than for an engine. “Basically, you have to keep contamination out of the system. The hydrogen must be pure; our standard is 99.999% pure coming into the stack,” says Freese. “We have to worry about contaminants that aren’t an issue in conventional power trains.”
Interestingly, the age of the fuel cell stack can also be a factor in its degradation. “Over time, air can intrude into parts of the stack. We have numerous ways of mitigating that effect, many of them proprietary,” says Freese. “Otherwise there can be reactions on one side of the stack that will tend to eat up the carbon support layers. Over long periods of time we can’t make that effect completely go away though we can reduce it.”
Another factor in aging is that the catalyst material can go through a change in morphology over time. “We want to keep the catalyst well distributed on active sites to minimize the amount of platinum used and maximize the reaction with gases. But the platinum tends to move over the life of the fuel cell stack. Platinum molecules can join with others to form bubbles and diminish the active surface area,” explains Freese. “The consequence of all those effects is that the amount of voltage the cell creates over time can deteriorate. So we have to oversize the cell a bit so it can produce enough power to run the vehicle at its end-of-life.”
Storing hydrogen
The usual way of storing hydrogen for fuel cells is to put it in a tank pressurized to about 10,000 psi. Perhaps the biggest challenge for fuel cell promoters is to convince consumers that it’s safe to put this high-pressure tank of hydrogen in a vehicle. The prospect of storing hydrogen under pressure has been contentious enough to spark the development of alternative storage schemes.
For example, Cella Energy in the UK has come up with an idea it calls solid-state hydrogen storage. The firm combines hydrogen with special plastic pellets which can be stored at ambient pressure. A vacuum pump moves batches of pellets to the cell. The pellets get heated to release the hydrogen. Spent pellets then get routed back to a storage tank again with a vacuum pump.
The difficulty with storing hydrogen in a material this way is that it requires energy to free the hydrogen for use by the fuel cell. “When you reliberate the hydrogen to get it into the stack it is easy to expend so much energy that you end up over-riding the advantages of the storage approach,” says Freese. “Sometimes you have to add mechanization on the vehicle that gives up the advantages of not using compressed gaseous storage.”
Some researchers have also stored hydrogen in liquid form as a way to avoid high pressure tanks. Liquid hydrogen stored near 50 psi has three times the energy per unit volume as gaseous hydrogen stored at 5,000 psi. The higher density means that about three times as much liquid hydrogen can be stored in about the same volume as 5,000-psi gaseous hydrogen. This is one reason the U.S. Naval Research Lab has been able to fly a 35-lb drone called the Ion Tiger for three days using liquid hydrogen fuel.
The big difficulty with liquid hydrogen is that it must be kept at temperatures near -423°F, so high-quality tank insulation is a must. That’s a problem when it comes to powering most vehicles. “Cryogenically stored hydrogen tends to warm up and boil off and must be vented. Cars spend a lot of time parked, and they could lose a lot of hydrogen just sitting. That’s why liquid hydrogen storage is just not a dominant path for vehicles,” says Freese.
It turns out that storing hydrogen under relatively high pressure is easier than to store it at cryogenic temperatures. “Industry has gone toward using a composite tank that tends to have a polymeric liner inside and an external carbon fiber wrap. They are really strong, and the carbon fiber is literally bullet proof. We shoot them with projectiles and they won’t puncture,” Freese says.
The more difficult obstacle for vehicle makers is fitting a hydrogen tank in the same space as a gas tank. “The optimum shape of a hydrogen tank is cylindrical. It just isn’t the same shape as gas tank. So we may have to go to using multiple tanks, each with an associated cost and complexity,” says Freese.
Inside a proton-exchange fuel cell
There are many chemistries that can go into fuel cells, but the most widely used is the hydrogen–oxide proton exchange membrane fuel cell design. Here a proton-conducting polymer membrane contains an electrolyte solution that separates the anode and cathode. On the anode side, hydrogen diffuses to the anode catalyst where it dissociates into protons and electrons. The protons conduct through the membrane to the cathode, but the electrons are forced to travel in an external circuit because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the protons and electrons which have traveled through the external circuit to form water.
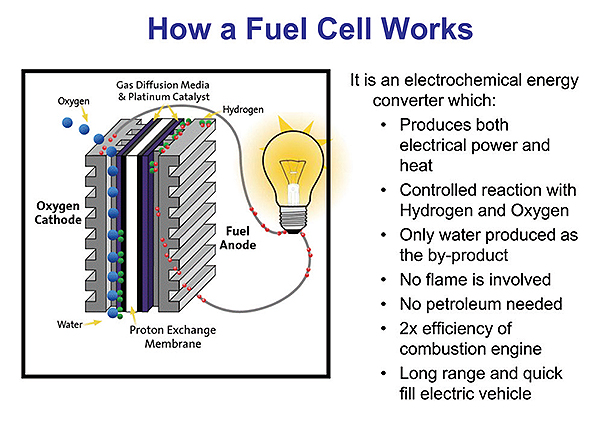
The components of the fuel cell include bipolar plates, electrodes, a catalyst, the proton exchange membrane, and ancillary hardware such as gaskets and current collectors. Bipolar plates have been made from numerous materials such as stainless steel, graphite, carbon composite, and 3D-printed power metal. The membrane electrode assembly is usually made of a proton exchange membrane sandwiched between two catalyst-coated carbon papers. Platinum usually serves as the catalyst.
References
Cella Energy
www.cellaenergy.com/
EnergyOR Technologies Inc.
www.energyor.com/
General Motors fuel cell research
www.gm.com/mol/m-2015-nov-1119-tardec.html
Intelligent Energy
www.intelligent-energy.com/
U.S. Naval Research Labs Ion Tiger fuel cell drone
http://tinyurl.com/jgv3l36


Leave a Reply