A little known chemical reaction helps extend battery life.
Sol Jacobs, Tadiran Batteries
As remote wireless devices become increasingly essential to the Industrial Internet of Things (IIoT), there is a growing need to understand the principles behind extended battery life. Many IIoT nodes require a battery-driven energy source.

These off-grid low-power applications fall into two categories. The first is those devices that draw average current measurable in microamps with high pulses measurable in the multi-amp range. Their annual energy consumption is low enough that an industrial-grade primary (non-rechargeable) battery is a practical power source. The second is made up of those devices that draw higher average amounts of energy (background current and pulses) measurable in milliamps. Here, the energy drain is enough to prematurely exhaust a primary cell, thus requiring use of an energy harvesting device combined with an industrial-grade rechargeable lithium-ion (Li-ion) battery to generate high pulses.
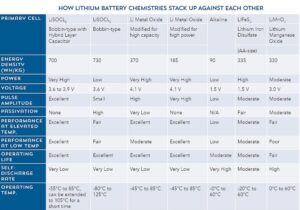
A point to note is that lithium batteries are not all alike. Lithium is the lightest non-gaseous metal. Lithium chemistries feature a high intrinsic negative potential that exceeds that of all other metals, offering the highest specific energy (energy per unit weight) and energy density (energy per unit volume) greater than any other chemistries. Lithium cells operate within a current voltage (OCV) range of 2.7 to 3.6 V. Lithium chemistry is also non-aqueous, thus less likely to freeze in extreme environments.

Within the lithium family there are numerous competing chemistries, including iron disulfate (LiFeS2), lithium manganese dioxide (LiMnO2), lithium thionyl chloride (LiSOCl2), and lithium metal-oxide, each offering unique advantages and disadvantages.
Of all these chemistries, LiSOCl2 is overwhelmingly chosen for long-term deployment in extreme environments, including AMR/AMI metering, M2M, SCADA, RFID, tank-level monitoring, animal and asset tracking, RFID, and environmental monitoring, and many other applications. Bobbin-type LiSOCl2 chemistry delivers the highest capacity and highest energy density of all lithium cells, which promotes product miniaturization.
 LiSOCl2 chemistry also features incredibly low self-discharge (less than 1% per year for certain cells), allowing certain low-power devices to operate up to 40 years on the original battery. Bobbin-type LiSOCl2 batteries also feature an extremely wide temperature range (-80 to 125°C), making them good candidates for use in extreme environments.
LiSOCl2 chemistry also features incredibly low self-discharge (less than 1% per year for certain cells), allowing certain low-power devices to operate up to 40 years on the original battery. Bobbin-type LiSOCl2 batteries also feature an extremely wide temperature range (-80 to 125°C), making them good candidates for use in extreme environments.
Bobbin-type LiSOCl2 batteries can be specially modified for use in the cold chain where consistent temperatures as low as -80°C must be maintained. Typical applications include the continuous monitoring during the transport of frozen foods, pharmaceuticals, tissue samples, and transplant organs. Bobbin-type LiSOCl2 batteries can also be modified for extremely high temperatures, including RFID tracking devices used in hospitals that must withstand many autoclave sterilization cycles at 125°C. Use of an extended-temperature battery saves time and money by eliminating the need to remove the battery prior to sterilization and also ensures 24/7 data continuity.
The importance of passivation
Self-discharge happens in all batteries as chemical reactions sap energy even while a battery is inactive or in storage. A battery’s self-discharge rate is impacted by numerous variables, including the cell’s current discharge potential, the purity and quality of raw materials, and the cell’s ability to harness the passivation effect.
Passivation occurs when a thin film of lithium chloride (LiCl) forms on the surface of the lithium anode to limit chemical reaction. Whenever a load is placed on the cell, the passivation layer also creates an initial high resistance, causing voltage to dip temporarily until the discharge reaction removes the passivation layer. This process keeps repeating each time the load is removed.
Passivation is affected by factors such as the current capacity of the cell, length of storage, storage temperature, discharge temperature, and prior discharge conditions; removing the load from a partially discharged cell can impact passivation relatively more than in a new cell. Passivation is essential for reducing self-discharge, but too much of it can also restrict energy flow when it is needed most. Reducing the level of passivation permits greater energy flow, but the trade-off is a higher self-discharge rate and shorter operating life.
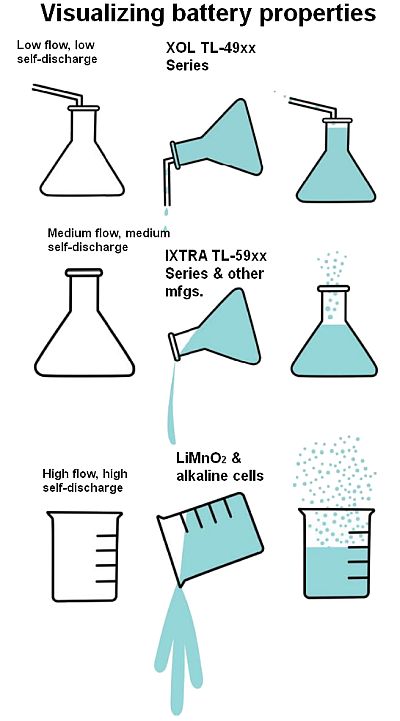
The effects of passivation on self-discharge and energy flow is analogous to fluid-filled bottles having different sized openings:
The volume of the glass/bottle is analogous to battery capacity
Evaporation/self-discharge is analogous to capacity loss
Flow volume is analogous to discharge/energy flow
Low liquid/electrolyte quality can clog the bottle opening, causing a stoppage to flow/passivation
Low liquid/electrolyte quality can cause evaporation/self-discharge
Large openings are good for fast flow/discharge but not for storing fluids for a long time
The ability to deliver a long life demands a smaller opening for low evaporation/self-discharge
Bobbin-type LiSOCl2 batteries have “small openings” (low flow rate with slower evaporation/self-discharge), while spiral-wound LiSOCl2, LiMnO2 and Alkaline cells have “larger openings” (higher flow rates with faster evaporation/self-discharge). The larger opening causes excessive evaporation/self-discharge. Conversely, an opening too small can get clogged (excess passivation), which can be exacerbated by chemical impurities.
Wireless devices increasingly require high pulses of short duration to power data queries and two-way wireless communications. Standard bobbin-type LiSOCl2 batteries deliver low-level background current during ‘stand-by’ mode but cannot generate high pulses because they are designed to provide low discharge rates. This challenge can be easily overcome with the addition of a patented hybrid layer capacitor (HLC) that delivers periodic high pulses. The HLC also offers an added bonus in the form of a unique end-of-life voltage plateau that can be interpreted to transmit low-battery status alerts.
Supercapacitors perform a similar pulse-supplying function for consumer electronics. But they are generally ill suited to industrial applications due to multiple drawbacks, including: linear discharge qualities that prevent use of all the available energy, low capacity, low energy density, and high annual self-discharge rates (up to 60% per year). Supercapacitors linked in series also require the use of cell-balancing circuits that add expense, bulkiness, and consume energy to accelerate their self-discharge rate.
Difficult to detect
The cumulative impact of high annual self-discharge (largely due to high passivation) may not become apparent for years. Unfortunately, predictive models used to determine theoretical battery life tend to be highly unreliable because they underestimate the effects of passivation and long-term exposure to extreme temperatures.
When ultra-long-life reliability is a concern, thorough diligence is in order when comparing brands. All potential battery suppliers should provide fully documented test results, in-field performance data under similar conditions, and multiple customer references.
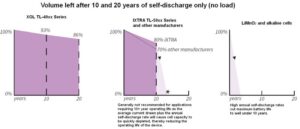
This due diligence is so important because a superior quality bobbin-type LiSOCl2 battery can feature a self-discharge rate as low as 0.7% per year, thus enabling certain low power devices to operate maintenance-free for up to 40 years. By contrast, a lower quality LiSOCl2 cell with a higher rate of passivation can exhaust up to 3% of its total energy each year, losing up to 30% of total capacity in 10 years, making 40-year battery life impossible.
All in all, a superior-quality battery can reduce or eliminate the need for future battery replacements to significantly lower the total cost of ownership.






Leave a Reply