A group of researchers have come up with an idea for an energy-harvesting battery that uses body fluids for part of its chemistry. In tests, device delivered an average power of 0.23 μW/mm2 of electrode area for an average of 6.1 days while sitting in the gastrointestinal tract of pigs. They say this power-harvesting cell could provide power to the next generation of ingestible electronic devices for prolonged periods of time inside the gastrointestinal tract.
The 12 researchers describe their work in a recent issue of the journal Nature Biomedical Engineering. Their battery cell consists of a redox couple formed by a dissolving metallic anode that undergoes galvanic oxidation and an inert cathode. Gastric or intestinal fluids form the electrolyte. They say the performance of the cell strongly depends on the environmental conditions that change significantly during normal gastrointestinal routines. For example, the pH, chemical composition and heterogeneity of the stomach contents vary a lot throughout the day. So researchers couldn’t learn anything by simulations; they had to make measurements while the battery sat in a real stomach.
Because the anodes dissolve in the stomach, they have to be comprised of materials that aren’t harmful. Magnesium and zinc are widely used choices because of their dietary value and inexpensive nature. The cathode, which sends electrons back into the solution, was created from pure copper metal. The problem with magnesium anodes, however, is that they corrode so quickly that the battery won’t last much longer than 24 hours. So the researchers settled on zinc anodes.
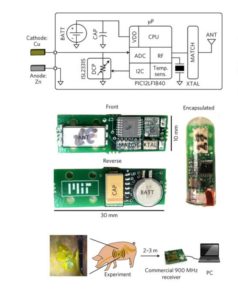
Researchers created a capsule able to measure how the Zn–Cu cell performed in the pig’s stomach and beam the results wirelessly to a nearby base station. A conventional coin-cell battery powered the capsule to avoid loading the electrodes. A separately controllable load was used to characterize how the battery cell behaved.
Pigs have a slow GI tract, so the capsule could stay in the animal transmitting data for seven to ten days. The mean time for which power was available, the mean peak power, and the mean voltage at mean peak power were 5.0 days, 1.14 μW/ mm2 and 0.149 V, respectively.
Researchers created a second capsule powered entirely by the Zn–Cu cell. This capsule measured temperatures and sent the data to a base station located 2 m away. It employed a commercial energy-harvesting boost-converter integrated circuit which took energy directly from the Zn–Cu cell at low voltage (0.1–0.2 V) and boosted it onto a temporary storage capacitor at a higher voltage (2.2–3.3 V) for use by the circuits.
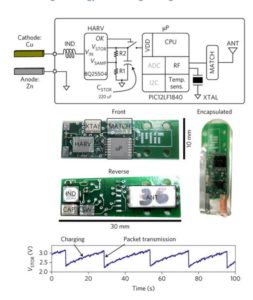
The capsule used a novel method of conserving energy. A microcontroller transmited packets containing temperature measurement data at a variable rate depending on the input power. The system regulates the rate by periodically sampling the supply voltage to determine whether to send a packet or wait for more energy to be harvested. If the sampled voltage is below 3.0 V, the system remains in a low-energy sleep mode for four seconds before attempting to sample again. If the voltage is above 3 V, the system transmits a packet and then returns to periodically sampling the voltage (initially 0.5 sec after the packet, and then again every four seconds) to determine when to transmit the next packet.
Researchers say the presence of the dissolving zinc in the stomach was a little like taking an over-the-counter zinc tablet. The total elemental zinc present in the largest electrode tested (30 × 3.0 × 0.25 mm) was 161 mg. Assuming the extreme case of full dissolution across the six-day experiment, the average zinc ion deposition rate for this electrode would be 27 mg/day. This amount is below the US Food and Nutrition Board recommended upper limit of 40 mg/day and in line with levels found in over-the-counter zinc supplements. Looking ahead, researchers say they would expect that a custom designed system would be able to target much lower power levels and hence integrate smaller electrodes and incur less zinc deposition.


Leave a Reply