Power-generation and heating units using radioactive decay as their primary energy source have been successfully used in space and on Earth for over 60 years.
Selecting a non-rechargeable (primary) battery to match the application is one of the many issues designers must contend with for the many situations in which recharging is neither feasible nor desirable. For these batteries, we must obviously consider basic electrical specifications such as battery voltage, energy capacity, and power delivery, of course.
This FAQ article will look at the use of radioactive decay to provide electrical power and heat for orbiting spacecraft and deeper-space missions, as well as terrestrial uses. This technique has been in use for over 60 years.
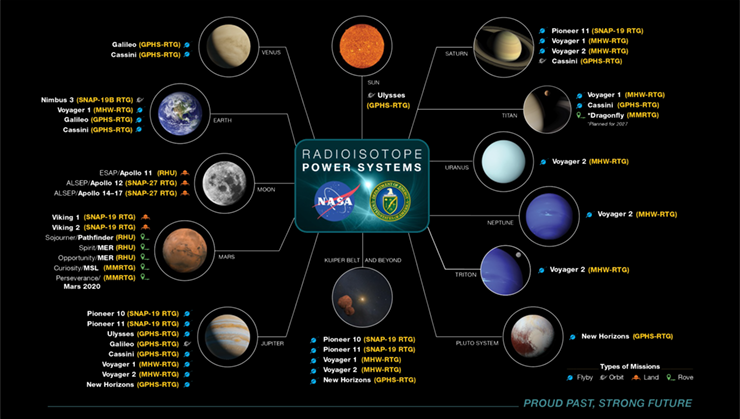
This technology has been used in nearly every space mission in some way — manned, unmanned, orbiting, and deep-space travel — since 1961 (Figure 1). It has demonstrated its performance, reliability, and consistency — all critical factors of these missions. It has also been used for remote Earth-based applications.
Q: What factors do system designers have to consider when choosing an electrochemical battery?
A: It often begins with identifying the appropriate battery chemistry, as each one has different attributes; among the choices are lithium-based, lead-acid, and silver oxide, along with many other chemistries and structures. Beyond the obvious battery output power and energy capacity is the unavoidable self-discharge rate even if the battery is disconnected; this can range from a few percent per year to around ten percent – and depending on the application time frame, that can compound and cause a steady, significant decrease.
Further, critical environmental conditions, including vibration and temperature extremes (hot and cold), affect battery life and performance. It’s important to keep in mind that a conventional battery is a component that stores energy in chemical form, so all the good and bad that comes with “chemistry” and “temperature” is part of the battery’s story and potential performance (pun intended).
Q: What are some of the applications of one-time primary cells?
A: Single-use battery applications range from mundane to advanced. They are used to trigger explosives in small throw-away gadgets, automotive remote key entry (RKE) fobs, burglar/fire alarm sensors/transmitters, scientific test instruments, remote monitoring stations, orbiting satellites, and planetary missions…the list goes on.
Despite all the attention that reachable batteries are getting, there are many cases where a non-rechargeable one is the only feasible solution, or at least the highly preferred one. Remember that a primary battery is a true energy source, while a second one is a hybrid that is part source, part repository.
Q: What’s unique about spacecraft?
A: Spacecraft have some extremely challenging requirements, of course. Their popular image shows them with solar panels and batteries to solve their power problem, with good reason. But the reality is also very clear: a highly efficient solar panel runs at 20%, which doesn’t count losses in power-conversion electronics. There just isn’t enough solar radiation for satellites that venture beyond Earth and maybe Mars to support a mission unless the needs are very limited.
Further, space radiation degrades the solar panels over time, as does cosmic “dust” settling on the panel surfaces. Even if the solar panels were adequate, most spacecraft still need a battery to get started and orient these panels and provide initial communication links, among other functions.
Q: But aren’t solar panels used extensively?
A: Yes, they are. They have been used for decades on small and large satellites such as the James Webb Space Telescope (about one million miles beyond Earth from the Sun) and even various Mars landers (average distance 142 million miles/228 million kilometers from the Sun). Still, in many cases, solar panels and the batteries they charge cannot provide the needed power.
Basic physics and math explain the reason. Solar energy diminishes as the square of the distance from the Sun. At Mars, it is only 43% of that at Earth. At Jupiter, it falls off to only 3.6% of Earth’s. By the time we get out to Pluto, solar energy is only .066% of what it is on Earth. Beyond the orbit of Mars, it is not practical to depend on solar power for a spacecraft.
Q: Are distance and degradation the only issues against solar panels and batteries?
A: No. Even when they can collect enough solar power and store it for recharging the batteries, there’s another absolutely unavoidable problem. As an electrochemical device, battery performance and life are affected by temperature extremes – and it’s cold in space.
How cold? The baseline “ambient” temperature of outer space, as set by the background radiation from the Big Bang, is 2.7 kelvins (−270 °C; −455 °F). Clearly, a chemical-based storage medium will not function at that temperature. Even spacecraft operating closer to the Sun, such as near Earth, endure thermal extremes, reaching hundreds of degrees C on their side facing the Sun, and supercold temperatures for the part facing away. It is possible to even this out somewhat by spinning the vehicle slowly combined with internal thermal management, but there are still hot/cold issues and problems.
Q: What can be done to use batteries in extreme cold?
A: For extreme cold, one option is to divert some of the precious battery power to heat itself, a waste of limited resources.
For self-discharge, you can get high-quality lithium-based primary batteries with extremely low self-discharge (such as those made by Tadiran), with a proven 30-to-40-year life when properly sized to the load. But they will not function in the extreme cold of space.
The next part looks at radioisotope-based heat and power principles and implementation.
Related EE World Content
Thermal electric generators, Part 1: Principles and Implementation
Thermal Electric Generators, Part 2: Applications
Gravity-assist “Slingshot,” Part 1: Background and principle
Gravity-assist “Slingshot,” Part 2: Application
Making sense of thermocouples and interfaces (Part 1)
External References (with some comments)
NASA, JPL, Dept. of Energy
- NASA/JPL, “Radioisotope power systems reference book for mission designers and planners” (Detailed, fact-packed 91-page book!)
- NASA/JPL. “A Look at the Next Generation of Radioisotope Thermoelectric Generators” (15 slides, excellent)
- NASA, “Cassini Radioisotope Thermoelectric Generators (RTGs)”
- NASA, “After 60 Years, Nuclear Power for Spaceflight is Still Tried and True”
- NASA, “RHU Pull-Apart Animation”
- NASA, “FAQ About RPS” (very useful)
- NASA, “A Step Forward in Reestablishing the Radioisotope Power Systems Supply Chain” (plutonium production issues)
- NASA, “Power Systems” (summary with video of RTGs)
- NASA, “Thermal Systems” (summary with video of RTUs)
- NASA, “NASA’s RHU-Heated and RTG-Powered Spacecraft” (broad overview)
- NASA, “So What’s an RTG and Are They Safe?
- NASA, “General Purpose Heat Source – Light-Weight Radioisotope Heater Unit”
- NASA, “RHU Fact Sheet”
- S. Department of Energy, “What is a Radioisotope Power System?”
- S. Department of Energy, “The History of Nuclear Power in Space”
- S. Department of Energy, “Overview of Light Weight Radioisotope Heater Unit (LWRHU) User’s Guide”
Other sources
- American Nuclear Society, Science and Technology Policy Institute,” Current Status and Future of Space Nuclear Power” (provides various technical and policy perspectives)
- Stanford University, “An Overview of Radioisotope Thermoelectric Generators”
- Wikipedia, “Radioisotope heater unit”
- Wikipedia, “Radioisotope thermoelectric generator”
Earth’s heat and radioactivity
- University of Notre Dame Nuclear Science Laboratory “Radioactivity, Lecture 11: The radioactive Earth”
- Physics World, “Radioactive decay accounts for half of Earth’s heat”
- Scientific American, “Do transuranic elements such as plutonium ever occur naturally?”
- Lawrence Berkeley National Laboratory “What Keeps the Earth Cooking?”





Leave a Reply