Engineers can choose between batteries, supercapacitors, or “best of both” hybrid supercapacitors for operating and backup power and energy storage.
Many systems operate from an available line-operated supply or replaceable batteries for power. However, in others, there is a need in many systems to continually capture, store, and then deliver energy to power the system. The amount of power can range from the minuscule amounts available via energy harvesting for IoT and remote-monitoring devices such as smart meters up through larger-scale grid-level systems. The situation is that it is one thing to immediately make use of energy from various sources in “real-time” as it is being generated or captured. Still, in the reality of an application, it is often necessary to have an energy-storage subsystem where any captured energy can be stored for later use.
This replenishable energy storage is often achieved through the use of rechargeable batteries (formally called secondary batteries, in contrast to primary, non-rechargeable batteries), or through the use of supercapacitors. This article will focus on supercapacitors after a brief look at batteries.
Begin with batteries
As is well known, batteries are electrochemical devices which provide energy storage and have been with us for hundreds of years. For many of these years, there have been two most widely used, common battery “chemistries”: the primary carbon-zinc devices and the rechargeable lead-acid, which really came into prominence with the development of the automobile electric starter around 1920.
In addition to these two chemistries, there are dozens of primary and secondary battery materials and chemistries in use. Each of these offers different characteristics for parameters such as terminal voltage, energy capacity, energy density by weight and volume, safety considerations, active discharge rates, self-discharge, operating temperature, shelf life, and cost, to cite just a few. There are also factors related to the optimal charging current and number of charge/discharge cycles (often called useful life).
For most smaller engineering designs and many larger ones, such as electric vehicles, the preferred rechargeable battery is based on lithium-based chemistry. There are many variations within this broad category, each offering different performance attributes, and tradeoffs.
However, one thing all rechargeable chemistries have in common is their limited number of useful charge/discharge cycles, which is on the order of one to several thousand such cycles, depending on how fully they are charged and how deeply they are discharged. This becomes a negative factor in repeatedly charged and discharged products, which is common in energy-harvesting and daily-cycling situations and so ultimately limits their usefulness.
Enter the supercapacitor
The supercapacitor is an excellent example of why you should “never say never” when it comes to technological advances. If you look in textbooks or academic papers about capacitors up to about the 1960s and even 1970s, there would be definitive statements on capacitors’ capacity limitations and physical size. Typically, after an explanation on the physics of capacitors and their energy capacity E:
E = ½ CV2
where C is the capacitance in farads (F), and V is the voltage, there would remarks that a capacitor on the order of one farad (F) would be impractically large, perhaps as large as a filing cabinet or small bookcase.
But research into materials and surface technologies led to new structures and fabrication techniques and eventually to what was dubbed the supercapacitor, providing tens and even hundreds of farads in a package comparable in size to other basic passives. The supercapacitor, also dubbed ultracapacitor, is formally called an electric double-layer capacitor (EDLC).
A classic capacitor has two conducting plates separated (no physical contact) and a dielectric between them; this dielectric can range from vacuum, to air, to non-conducting polymers.
The construction of the supercapacitor is simple looking but more complicated. Supercapacitors use carbon nanotube technology to create a very large surface area with an extremely small separation distance (Figure 1). They do not have the traditional dielectric material such as ceramic, polymer films, or aluminum oxide to separate the electrodes but instead have a physical barrier made from activated carbon such that when an electrical charge is applied to the material, a double electric field is generated which acts as a dielectric.
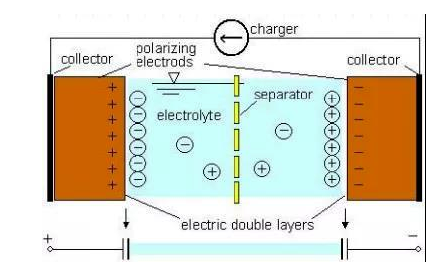
The thickness of this electric double layer is as thin as a molecule. In contrast, the surface area of the activated carbon layer is extremely large, as high as several thousands of square meters per gram. The large surface area supports the absorption of a large amount of ions. Thus, the charging/discharging occurs in an ion absorption layer formed on activated carbon electrodes. The electric double layer effect occurs at the interface between the electronic conductor and the ionic conductor, which is present in almost all electrochemical energy storage systems and involves what are called Helmholtz layers.
The next part of this article looks at supercapacitors in more detail and then explores the hybrid (battery plus supercapacitor) in more detail.
EE World Related Content
Supercapacitor line now ranges from coin cell, radial form factors, large snap-in types
Supercapacitors operate at 3 and 6 V
What’s new in electrolytic capacitors and supercapacitors for energy harvesting
Supercapacitor system design considerations
Supercapacitor specifications and lifetimes – Virtual Roundtable (part 2 of 2)
Supercapacitor ESR and optimal performance – Virtual Roundtable (part 1 of 2)
Supercapacitor specifications and IEC/EN 62391–1
Supercapacitor operation – a higher power source
Applying large banks of supercapacitors
External References
Wikipedia, “Supercapacitor
Eaton, “Hybrid supercapacitors explained
Eaton, “HS Hybrid supercapacitor white paper
Eaton, “Supercapacitor back up power solutions”
Eaton, “HB Supercapacitors Cylindrical cells” (supercapacitor data sheet)
Eaton, “HS/HSL Hybrid Supercapacitors” (hybrid supercapacitor data sheet)
Battery University (Cadex), “BU-209: How does a Supercapacitor Work?”
Taiyo Yuden, “Lithium Ion Capacitors: The Ultimate EDLC Replacement”
Taiyo Yuden, “Power Storage Devices: Lithium Ion Capacitors;Electric Double-Layer Capacitors”
Tech Briefs, “Supercapacitors Go Hybrid for Increased Performance and Efficiency
Kemet, “Supercapacitors vs. batteries”
Illinois Capacitor, “Supercapacitors”
Basic Electronics 16, “Supercapacitors”
Jinzhou Kaimei Power Co., Ltd. “The Supercapacitors: its Basic Principles, Classification, and its Electrical Performance”






Leave a Reply